MENLO PARK, Calif., Jan. 30, 2025 (GLOBE NEWSWIRE) — A groundbreaking precision medicine system developed by biotech trailblazer Diag-Nose.io is set to transform the management of chronic respiratory diseases worldwide after closing its seed round this week.
Diag-Nose.io founding team at the Stanford EENT Innovation Program
A graduate of Stanford University’s EENT Innovation Biodesign program in 2019, Diag-Nose.io is pioneering advancements in respiratory healthcare by focusing on the “unified airway”: the biological relationship between the lungs and nasal passages.
Traditional diagnostic methods for chronic respiratory conditions – such as asthma chronic obstructive pulmonary disorder (COPD) and chronic sinusitis – rely on limited measurement tools and subjective assessments. These flawed processes currently result in treatment failure for more than 30% of patients, costing hundreds of millions of dollars per year.
Using machine learning (artificial intelligence) and protein signatures, Diag-Nose.io’s RhinoMAP™ aims to remove the guesswork, enabling providers to match treatments to a patient’s unique biological profile, or “endotype.”
Diag-Nose.io’s approach involves collecting nasal samples using the ABEL Microsampler™, its patented nasal liquid biopsy device, followed by advanced analysis through the AI-powered RhinoMAP™ platform. The system provides insights into disease progression, therapeutic responsiveness, and triage capabilities.
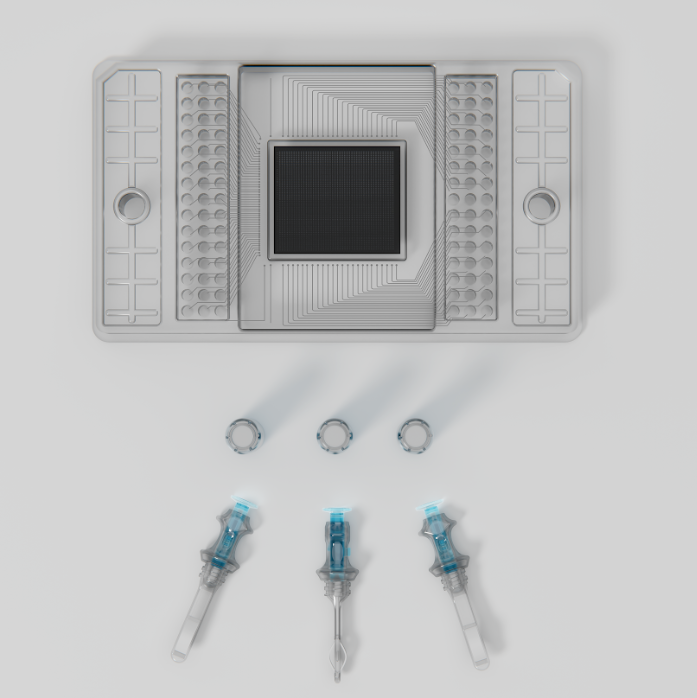
RhinoMAP platform
“This funding allows us to delve deeper into the unexplored biology of respiratory diseases and unlock novel therapeutic pathways,” said Dr. Josie Xu, Co-Founder of Diag-Nose.io and former otolaryngology and sleep apnea surgery fellow at University of Southern California Keck School of Medicine. “By combining innovative nasal liquid biopsy techniques with proteomics and AI-driven analysis, we aim not only to enhance the adoption of current treatments but also to pave the way for future breakthroughs in areas such as sleep disorders and beyond.”
The oversubscribed $2 million round was led by Breakthrough Victoria, with additional investments from Radar Ventures, a syndicate of physicians from Specialty Physician Associates (SPA), biotech angel investors Carl Stubbings, Stephen Ho, and Richard Lipscombe, as well as physicians affiliated with leading U.S. hospitals. Carl Stubbings will join Diag-Nose.io’s board as Chairperson.
“I have closely and proudly followed Diag-Nose.io’s journey since they graduated from the Stanford EENT Innovation program in 2019. The team has not only demonstrated impressive scientific progress despite the pandemic, but they have also grown increasingly ambitious.” said Professor Robson Capasso, Chief of Sleep Surgery, Professor of Otolaryngology and Head and Neck Surgery, and Associate Dean of Research at Stanford University School of Medicine.
“It makes them well-positioned to tackle one of the burning problems in respiratory care right now; matching patients to the right biologic therapy.”
Diag-Nose.io plans to launch a series of clinical studies across the United States in 2025 to further validate its technology and invites interested academic and industry partners to collaborate in driving forward this revolutionary approach to respiratory care.
MEDIA CONTACT
For any questions in relation to this release or to discuss interviews, please contact:
Nicole Papoutsis
media@diag-nose.io
About Diag-Nose.io
Diag-Nose.io, founded in 2020, is a biotechnology company focused on translating the complexities of the unified airway into precision diagnostic and drug discovery solutions.
Their precision medicine technology combines advanced proteomics, computational biology, and AI (machine learning) to create a scalable respiratory biology model. This innovation aims to help clinicians prescribe the right treatments faster and enable researchers to accelerate the development of new therapies.
The company’s flagship platform, RhinoMAP™, leverages proteomic data to predict respiratory disease activity, monitor therapy response and predict treatment efficacy in advance, with an initial focus on anti-Th2 biologics.
website: https://diag-nose.io/
Disclaimer:
The RhinoMAP platform, ABEL microsampler™, RhinoMAP™ and any associated technologies or products developed by Diag-Nose.io have not been evaluated by the U.S. Food and Drug Administration (FDA) and are not currently approved for diagnostic or therapeutic use.
This press release is for informational purposes only and should not be interpreted as medical advice. Consult a qualified healthcare professional for medical guidance.
This announcement does not constitute an offer to sell, or a solicitation of an offer to buy, securities in the United States or any other jurisdiction. Any securities described in this announcement have not been, and will not be, registered under the US Securities Act of 1933 and may not be offered or sold in the United States except in transactions exempt from, or not subject to, the registration of the US Securities Act and applicable US state securities laws.
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/df9ff174-42a7-4a7f-b1f7-23a2bc80274c
https://www.globenewswire.com/NewsRoom/AttachmentNg/0f45d27e-6518-4a11-9185-b3182139249f
