- First Patient Expected to be Dosed Next Week with Intranasal Foralumab
- PET Scan Shows Presence of Untreated Neuroinflammation in Alzheimer’s Patient on Leqembi® (Lecanemab) Anti-Amyloid Therapy
BOSTON, Dec. 12, 2025 (GLOBE NEWSWIRE) — Tiziana Life Sciences, Ltd. (Nasdaq: TLSA) (“Tiziana” or the “Company”), a biotechnology company developing breakthrough immunomodulation therapies with its lead development candidate, intranasal foralumab, a fully human, anti-CD3 monoclonal antibody, announces that enrolment has begun in its Phase 2 randomized, placebo-controlled early Alzheimer’s clinical trial and plans to dose the first patient next week.
The Phase 2 clinical trial will evaluate intranasal foralumab both as monotherapy and in combination with an FDA approved anti-amyloid therapy, lecanemab or donanemab, in patients with early Alzheimer’s disease (AD). Baseline clinical assessments, cognitive testing, TSPO-PET imaging, and fluid biomarkers have been completed in the first participants screened in the trial.
The clinical trial launch is supported by new TSPO-PET imaging evidence demonstrating persistent and widespread microglial activation in an Alzheimer’s patient despite treatment with lecanemab, confirming that neuroinflammation remains present even after amyloid plaque reduction. Lecanemab, marketed by Eisai and Biogen as Leqembi®, is one of the two FDA-approved anti-amyloid therapies for treating early Alzheimer’s and is proven to reduce beta-amyloid plaques.
Figure 1. TSPO-PET scan of an Alzheimer’s patient treated with lecanemab demonstrating persistent and widespread microglial activation throughout the brain.
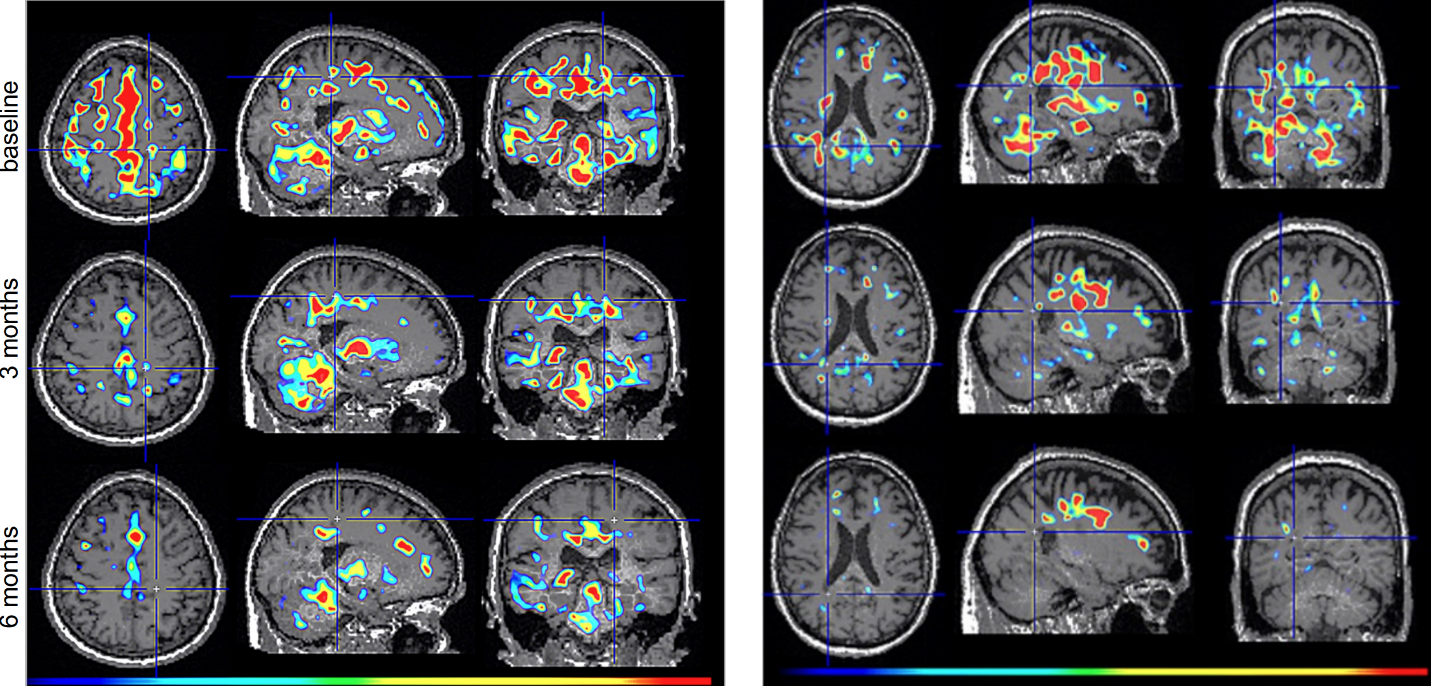
Dr. Howard Weiner, Chairman of Tiziana’s Scientific Advisory Board and co-director of the Ann Romney Center for Neurologic Diseases at Brigham and Women’s Hospital, a founding member of Mass General Brigham, stated, “This PET finding is a critical insight: clearing amyloid does not turn off the brain’s inflammatory response. We believe intranasal foralumab directly addresses this residual neuroinflammation by inducing regulatory T cells to migrate to the brain and calm activated microglia — a mechanism we have already shown reduces microglial activation in secondary progressive multiple sclerosis.”
Figure 2. TSPO-PET scans from intranasal foralumab-treated MS patients showing marked reduction in microglial activation over a 6-month treatment period.
Phase 2 Trial Design and Rationale
The study will evaluate whether intranasal foralumab:
- As a monotherapy, reduces microglial activation and slows cognitive decline by suppressing neuroinflammation.
- In combination with lecanemab or donanemab will provide additive or synergistic benefit by simultaneously targeting amyloid pathology and persistent microglial inflammation.
“With enrolment now underway and baseline neuroimaging and biomarkers secured, we are on the verge of testing a fundamentally new approach to Alzheimer’s — one that treats the chronic brain inflammation that is associated with ongoing neurodegeneration. We expect the first patients to begin dosing within days,” said Ivor Elrifi, Chief Executive Officer of Tiziana Life Sciences. “This milestone marks an important step toward testing whether immune modulation alone, or in combination with amyloid removal, can achieve disease modification. The PET evidence of inflammation in AD with or without anti-amyloid treatment provides a clear biological rationale for this innovative strategy.”
Intranasal foralumab is a fully human anti-CD3 monoclonal antibody designed to induce Tregs and suppress pathological neuroinflammation, positioning it as a potential first-in-class therapy to address the inflammatory component of Alzheimer’s disease that remains active even after amyloid clearance.
About the Phase 2 Trial
The Phase 2 study is designed to evaluate the safety, tolerability, and efficacy of intranasal foralumab as both a monotherapy and in combination with lecanemab or donanemab in early/mild AD. Key endpoints will include measures of neuroinflammation (via TSPO-PET imaging), cognitive function, and biomarker changes related to amyloid and tau pathology. Results from this trial will inform future development and may establish a new paradigm for treating AD by targeting neuroinflammation alone or in combination with anti-amyloid therapy.
About Foralumab
Foralumab, a fully human anti-CD3 monoclonal antibody, is a biological drug candidate that has been shown to stimulate T regulatory cells when dosed intranasally. At present, 14 patients with Non-Active Secondary Progressive Multiple Sclerosis (na-SPMS) have been dosed in an open-label intermediate sized Expanded Access (EA) Program (NCT06802328) with either an improvement or stability of disease seen within 6 months in all patients. In addition, intranasal foralumab is currently being studied in a Phase 2a, randomized, double-blind, placebo-controlled, multicenter, dose-ranging trial in patients with non-active secondary progressive multiple sclerosis (NCT06292923).
Foralumab is the only fully human anti-CD3 monoclonal antibody (mAb) currently in clinical development. Immunomodulation by intranasal foralumab represents a novel avenue for the treatment of neuroinflammatory and neurodegenerative human diseases.[1],[2]
About Tiziana Life Sciences
Tiziana Life Sciences is a clinical-stage biopharmaceutical company developing breakthrough therapies using transformational drug delivery technologies to enable alternative routes of immunotherapy. Tiziana’s innovative intranasal approach has the potential to provide an improvement in efficacy as well as safety and tolerability compared to intravenous (IV) delivery. Tiziana’s lead candidate, intranasal foralumab, which is the only fully human anti-CD3 mAb currently in clinical development, has demonstrated a favorable safety profile and clinical response in patients in studies to date. Tiziana’s technology for alternative routes of immunotherapy has been patented with several applications pending and is expected to allow for broad pipeline applications.
For more information about Tiziana Life Sciences and its innovative pipeline of therapies, please visit www.tizianalifesciences.com.
Forward-Looking Statements
Certain statements made in this announcement are forward-looking statements. These forward-looking statements are not historical facts but rather are based on the Company’s current expectations, estimates, and projections about its industry, its beliefs, and assumptions. Words such as ‘anticipates,’ ‘expects,’ ‘intends,’ ‘plans,’ ‘believes,’ ‘seeks,’ ‘estimates,’ and similar expressions are intended to identify forward-looking statements. These statements are not guarantees of future performance and are subject to known and unknown risks, uncertainties, and other factors, some of which are beyond the Company’s control, are difficult to predict, and could cause actual results to differ materially from those expressed or forecasted in the forward-looking statements. The Company cautions security holders and prospective security holders not to place undue reliance on these forward-looking statements, which reflect the view of the Company only as of the date of this announcement. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: the uncertainties related to market conditions and other factors described more fully in the section entitled ‘Risk Factors’ in Tiziana’s Annual Report on Form 20-F for the year ended December 31, 2024, and other periodic reports filed with the Securities and Exchange Commission. The forward-looking statements made in this announcement relate only to events as of the date on which the statements are made. The Company will not undertake any obligation to release publicly any revisions or updates to these forward-looking statements to reflect events, circumstances, or unanticipated events occurring after the date of this announcement except as required by law or by any appropriate regulatory authority.
For further inquiries:
Tiziana Life Sciences Ltd
Paul Spencer, Business Development, and Investor Relations
+44 (0) 207 495 2379
email: [email protected]
[1] https://www.pnas.org/doi/10.1073/pnas.2220272120
[2] https://www.pnas.org/doi/10.1073/pnas.2309221120
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/48f529f2-1fc4-4466-ae1d-84fa808979a8
https://www.globenewswire.com/NewsRoom/AttachmentNg/bc4d6ed6-2598-489a-859a-f4387ad81ba0

