- Preclinical data continues to demonstrate RECCE® 327’s significant bactericidal activity against Neisseria gonorrhoeae (N. gonorrhoeae), with a 4-log (99.99%) and 3.5-log (>99.9%) reduction
- N. gonorrhoeae is listed as a priority pathogen on the World Health Organization’s (WHO) list of bacteria that poses a significant threat to human health
SYDNEY, Australia, Dec. 18, 2023 (GLOBE NEWSWIRE) — Recce Pharmaceuticals Ltd (ASX: RCE, FSE: R9Q) (the Company), the Company developing a new class of synthetic anti-infectives, today announced positive preclinical results from an in vivo study evaluating the antibacterial efficacy of its lead synthetic anti-infective candidate, RECCE® 327 (R327), against Neisseria gonorrhoeae (N. gonorrhoeae) in a mouse vaginal infection model.
“The need for a new class of anti-infectives could not be greater, especially against a sometimes lethal pathogen, such as N. gonorrhoeae,” said James Graham, Chief Executive Officer of Recce Pharmaceuticals. “The data from this study, along with the previous findings, emphasize the capability of R327 to demonstrate broad spectrum activity against antibiotic-resistant bacteria, even with repeated use.”
In the study, groups of 10 mice were inoculated vaginally with N. gonorrhoeae. R327 was administered twice daily as an intravenous (IV) bolus dose of 1,000mg/kg. After three days, the mice treated with R327 showed an approximate 4-log (99.99%) reduction in bacterial shedding, demonstrating significant bactericidal activity. After five days of treatment, R327 showed a 3.5-log (>99.9%) reduction in bacterial shedding compared to the placebo-treated group.
By the end of the treatment period, R327 significantly reduced vaginal gonococcal shedding. In this study, a two-log reduction, equivalent to a 99% reduction in bacterial burden, is commonly considered a significant effect. The mice displayed no clinical signs of gonococcal infection.
In a previous in vivo study against gonorrhea conducted by an independent contract research organization, R327 showed a significant dose-dependent antibacterial effect in vaginal load at 100, 500, and 1,000 mg/kg given as an IV bolus dose twice daily for seven days when compared to the vehicle-infected control group seven days post-infection. The recognized vaginal infection model met its primary endpoint of a reduction in bacterial load compared to vehicle-infected control evaluated on the seventh day following dosing.
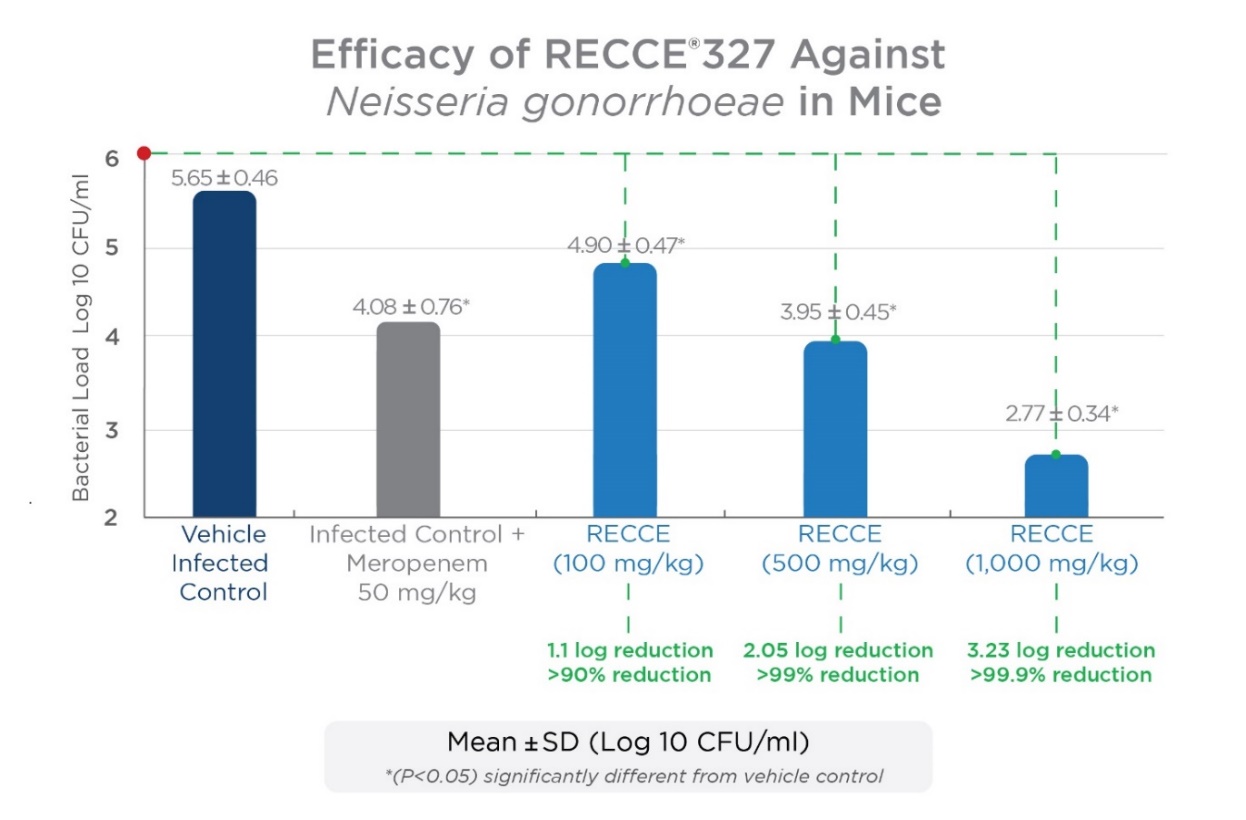
The IV administration of R327 further emphasizes its systemic potential to treat a wide range of bacterial infections. A late-stage preclinical study comparing IV administration to a topical application of R327 against N. gonorrhoeae is underway. R327 has demonstrated a positive safety and tolerability profile in clinical trials to date, supporting the potential to progress to Phase II studies if preclinical studies are successful.
Antimicrobial resistance to gonorrhea is a serious and growing problem, rendering many classes of antibiotics ineffective with the risk of becoming untreatable.1 Current treatment involves combination therapy using at least two antibiotics (ceftriaxone and azithromycin); however, bacterial resistance has recently led to restriction for infections caused by resistant organisms.
The World Health Organization (WHO) has recognized gonorrhea as a significant public health problem that is on the rise worldwide, with strains resistant to many antibiotics emerging. In 2020, the WHO estimated 82.4 million new infections with N. gonorrhoeae among adults aged 15 to 49.
About Recce Pharmaceuticals Ltd
Recce Pharmaceuticals Ltd (ASX: RCE, FSE: R9Q) is developing a New Class of Synthetic Anti-Infectives designed to address the urgent global health problems of antibiotic-resistant superbugs and emerging viral pathogens.
Recce’s anti-infective pipeline includes three patented, broad-spectrum, synthetic polymer anti-infectives: RECCE® 327 as an intravenous and topical therapy that is being developed for the treatment of serious and potentially life-threatening infections due to Gram-positive and Gram-negative bacteria including their superbug forms; RECCE® 435 as an orally administered therapy for bacterial infections; and RECCE® 529 for viral infections. Through their multi-layered mechanisms of action, Recce’s anti-infectives have the potential to overcome the hypercellular mutation of bacteria and viruses – the challenge of all existing antibiotics to date.
The FDA has awarded RECCE® 327 Qualified Infectious Disease Product designation under the Generating Antibiotic Initiatives Now (GAIN) Act – labelling it for Fast Track Designation, plus 10 years of market exclusivity post approval. Further to this designation, RECCE® 327 has been included on The Pew Charitable Trusts Global New Antibiotics in Development Pipeline as the world’s only synthetic polymer and sepsis drug candidate in development. RECCE® 327 is not yet market approved for use in humans with further clinical testing required to fully evaluate safety and efficacy.
Recce wholly owns its automated manufacturing, which is supporting present clinical trials. Recce’s anti-infective pipeline seeks to exploit the unique capabilities of its technologies targeting synergistic, unmet medical needs.
Corporate Contact
James Graham
Recce Pharmaceuticals Ltd
+61 (02) 9256 2571
[email protected]
Media & Investor Relations (AU)
Andrew Geddes
CityPR
+61 (02) 9267 4511
[email protected]
Media (USA)
Jordyn Temperato
LifeSci Communications
[email protected]
Investor Relations (USA & EU)
Guillame van Renterghem
LifeSci Advisors
[email protected]
________________________________________________________________
1 https://www.who.int/news-room/fact-sheets/detail/gonorrhoea-(neisseria-gonorrhoeae-infection)
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/6b66caf4-c066-411c-83ef-cca0810eb8cd
https://www.globenewswire.com/NewsRoom/AttachmentNg/8dcda488-b143-47fb-a5f5-05ddd3fcc204

