University of Nottingham
MSc Development Economics
Master’s Dissertation
Impact of the Child Support Grant on Nutritional Outcomes
in South Africa:
Is there a ‘pregnancy support’ effect?
Author: Supervisor Claire Lynam Trudy Owens
Student Number:
20398197
This Dissertation is presented in part fulfilment of the requirement for the completion of
an MSc in the School of Economics, University of Nottingham. The work is the sole
responsibility of the candidate.
Abstract
Research has long emphasised a ‘critical window’ for nutrition within the first 1000 days of a child’s life, of which the first 270 occur during pregnancy. Current literature on infant health in South Africa focuses on the post-birth period of this window, failing to account for the importance of care while in utero for neonatal health outcomes. Poorly nourished mothers are more likely to give birth to underweight babies, increasing a child’s probability of being stunted. This paper demonstrates the first robust evidence of a ‘pregnancy support’ spillover effect, where mothers who reside in Child Support Grant (CSG) recipient households provide extra nourishment to subsequent children in utero. Recipients of pregnancy support show significant gains in height-for-age standardised scores (HAZ). At the same time, a lack of support hosts limited potential for catch-up growth and highlights the value of prenatal intervention. Average treatment effects are computed by Augmented Inverse Probability Weighted (AIPW) estimators and household Fixed Effects regressions, using the National Income Dynamic Survey (NIDS) data Waves 1 to 5. The existence and importance of pregnancy support spillovers are twofold: current South African literature overlooks this effect, potentially underestimating the true impact of the CSG. Moreover, the additional gains from prenatal treatment provide nuanced policy insight into the benefits of expanding the current post-delivery CSG into pregnancy.
1 Introduction
With remarkably high rates of child stunting, South Africa bears a large burden of hindered development potential in children (Shonkoff et al. 2012, Black et al. 2013, Casale 2020). Analysis of child nutrition is commonly framed in terms of long-term consequences of poor health: lesser cognitive ability, lower potential earnings and the persistence of inter-generational poverty and poorer foetal health (Hoddinott et al. 2008, Victora et al. 2008, Dewey & Begum 2011, Casale et al. 2014). Accordingly, since 1998, the South African government has offered a Child Support Grant (CSG) that aims to alleviate part of the socio-economic burden of children in low-income households while improving health outcomes and general child welfare
Existing evidence shows that the CSG has effectively improved children’s nutritional outcomes among beneficiary families (Aguero et al. 2006, Coetzee 2013). This grant is offered as an unconditional cash transfer, with families eligible after the birth of a child. There is thus the possibility for a spillover ‘pregnancy support’ effect: after mothers receive a CSG for their first child, any subsequent children may be better nourished during pregnancy because of CSG spillovers. The existence of a pregnancy support effect is important as current evidence suggests that the first 1000 days of a child’s life, including months in-utero, is a critical period where conditions can have persistent and long-term effects on health and cognition (Barker 1990, de Rooij et al. 2010, Andersen 2003, Thompson & Nelson 2001, Chang et al. 2022)
This paper therefore examines whether the ‘pregnancy support’ effect exists as a result of the CSG. Nonexperimental methods are applied to five waves of data from the National Income Dynamics Study (NIDS) in South Africa. To control for selection effects in the programme, this paper applies the Augmented Inverse Probability Weighted (AIPW) estimator to both cross-sectional and panel data and utilises a household Fixed Effects regression estimation.
Average treatment effects show that the pregnancy support effect exists within the CSG: children who received support while in utero have, on average, improved height-for-age standardised scores (HAZ) of 1.76 standard deviations and higher birthweights by 43 grams, compared to children in comparable households who did not receive extra nourishment. These results are corroborated by considering differences between firstborn and second born siblings, where the additional gain from prenatal intervention for second children (compared to post-delivery CSG receipt for first children) is statistically significant and large. Not only does the pregnancy support effect exist, but the impact for untreated children persists into later childhood. Children who do not receive prenatal support are unable to catch up the additional HAZ reported by the treated group1 , even if receiving the CSG from very young ages.
These findings have both current and future implications. The existence of the
¹Catch up growth is measured approximately two years after post-treatment
estimates.
pregnancy support effect corroborates that households pool income (including grant income) in South Africa. Mothers receiving child support for one of their children are able to improve birth outcomes for subsequent children. Existing literature illustrates the benefits of CSG receipt and the additional gains from early uptake. The findings in this paper build on these outcomes by suggesting that current literature underestimates the true effect of the CSG by overlooking the pregnancy support effect. Thus, this paper demonstrates an even greater importance of CSG uptake and highlights the marginal gains for early intervention starting during pregnancy (rather than after birth). Given the high returns to early investment in nutrition and the long-term consequences for human development as a result of stunting (defined by a HAZ less than -2), the potential multiplier effect for a cash transfer directed at pregnant women is expectantly large. To preclude the pregnancy period from the CSG is an inefficient use of resources, undermining current social protection measures that are already in place.
The rest of the paper is structured as follows. Section 2 presents a brief literature review, after which Section 3 explores the Child Support Grant, conceptualises the pregnancy support treatment effect and provides a description of the NIDS data used for this analysis. In Section 4, the key identification problem is explained, with a na¨ıve estimation of this research question suggesting there is certain impact of pregnancy support. Section 5 outlines the empirical specifications used for this analysis, which are then applied in Section 6. Robustness checks are performed in Section 7 to validate the results, with alternative expansions considered for a comprehensive understanding given the novel nature of this effect. The paper concludes in Section 8, drawing attention to potential policy implications from these findings
2 Review of Literature
Combatting poor nutrition in early childhood is one of the most significant development challenges around the world (UNICEF 2012, World Bank 2006). Good nutrition underpins child survival, wellness and growth, and allows children to better partake in and contribute to their communities (Waidler & Devereux 2019, Casale et al. 2014, Caulfield et al. 2004). Globally, malnutrition is the underlying cause of half of all child deaths (Devereux et al. 2019, Caulfield et al. 2004), increasing to 60 per cent in developing countries (Zembe-Mkabile et al. 2016). UNICEF (2012) reports 155 million chronically malnourished children under the age of five (World Health Organization 2015), of which 56.6 million are in Africa, and 1.5 million are in South Africa. These estimates suggest that one in four children under five suffer from chronic malnourishment in South Africa (Devereux et al. 2019, May & Timaeus 2014).
South Africa offers a post-delivery Child Support Grant, which provides cash transfers to means eligible mothers after the birth of a child (see Section 3.1 for details about the grant). Coetzee (2013) uses the first wave of the South African National Income Dynamic Survey (NIDS) data, collected in 2008, to estimate the effect of the CSG on child health, nutrition and education. Initially examining a binary treatment for CSG receipt, she applies propensity score matching to two nonexperimental evaluation techniques and finds no significant effects. Alternatively, following Hirano & Imbens (2004) and applying a generalised form of the propensity score, Coetzee (2013) estimates positive, albeit small, treatment effects found for children receiving the CSG. A similar approach is taken by Aguero et al. (2006), who examine the impact of the CSG on children in their first thirty-six months after birth. Using the KwaZulu-Natal Income Dynamics Study (KIDS), this study finds a more significant impact of the CSG on child HAZ than Coetzee (2013), as well as marginally improved health outcomes over extended periods of CSG receipt.
While the CSG has been effective in improving recipient children’s nutritional status, it currently precludes direct support for women during pregnancy. Extensive literature emphasises a ‘critical window’ for good health during the first 1000 days of a child’s life, with the first 270 days occurring during pregnancy (Casale 2020, Casale et al. 2014, Norman et al. 2007). A mother’s nutritional status during this period is a critical determinant of her child’s birthweight and longer-term health (Norman et al. 2007, Gonz´alez & Trommlerov´a 2022), with nutritional deprivation increasing her chance of maternal morbidity and risking lifelong impairment for child development (Norman et al. 2007, UNICEF 2012, Black et al. 2013, Qadir & Bhutta 2009, World Health Organization 2015). These consequences are persistent, with research in South Africa and other developing countries finding a strong inter-generational transmission (Aguero et al. 2006, May & Timaeus 2014, Paxson & Schady 2005, Katepa-Bwalya et al. 2015)
Given the link between poor foetal nutrition and reduced human capital development, as well as the association between intrauterine growth restriction2 and stunting (Danaei et al. 2016), providing income support to pregnant mothers is likely to reduce South Africa’s high burden of stunting and improve general child health outcomes (Grow Great 2021). Although South Africa lacks a grant purely intended for pregnancy, existing social protection may hold benefits for children in utero if grant income has spillover effects within households. Pooling of grant income has been found for the CSG (Coetzee 2013) and other grants, such as the Old Age Pension programme (Duflo 2003), whereby individual grant receipt has shared household benefits. Thus, mothers residing in CSG recipient households during pregnancy are plausibly able to provide better nourishment to children in utero, who benefit from this spillover pregnancy support effect. To date, this effect has not been investigated. In South Africa, scarce literature reports the effect of cash transfers to mothers during pregnancy (Chersich et al. 2016). However, the potential effect is large, with lower risks of birth complications for healthy mothers, reduced healthcare costs and services for healthy children, and potential economic growth and development given a reduced burden of stunting (Grow Great 2021). Where empirical literature exists,
²Intrauterine growth restriction occurs when a foetus in the womb does not grow
as expected and is not as big as expected, given the stage of pregnancy.
the cash transfers examined are provided by donors, making the sample size small and restricting studies to single geographic areas. Therefore, the income effect in these papers bears no association with current social protection programmes (Grow Great 2021, Chersich et al. 2016).
Globally, a larger body of research considers the impact of cash transfers on child health in utero (Gonz´alez & Trommlerov´a 2022, Amarante et al. 2016). While the CSG targets children, most social protection programmes are targeted toward vulnerable households, allowing for a more straightforward evaluation of newborn health outcomes. Studying a generous social assistance programme in Uruguay, Amarante et al. (2016) report that participation in the programme reduces the incidence of low birthweight for children, weakening the cycle of inter-generational poverty. Similarly, a study by Gonz´alez & Trommlerov´a (2022) exploits the unexpected introduction of universal health benefits in Spain to estimate the impact of cash transfers targeting new mothers on their subsequent children’s health outcomes at birth. Although regression discontinuity methods are applied, the study plausibly asks the most similar research question to this paper. Gonz´alez & Trommlerov´a (2022) find that women who receive treatment are far less likely to have children with low birthweight in the future and that poor, unmarried woman with low educational attainment benefit the most.
3 Conceptual Framework and Data.
3.1 The South African Child Support Grant
The South African CSG was introduced in April 1998 to benefit children in vulnerable households. This grant is considered one of the government’s most successful anti-poverty interventions (UNICEF 2012, Coetzee 2013), with the post-century decline in poverty levels primarily attributed to its introduction (Woolard & Leibbrandt 2013, Aguero et al. 2006).
Grant eligibility is determined by a means test for the primary caregivers of children3 , who receive the grant on a child’s behalf (Mackett 2020). Between 1998 and 2008, the means test remained unchanged, with caregivers who earned below R800 in urban areas, and R1100 in rural areas deemed eligible for CSG receipt. The means test changed in 2008, to differentiate between single and married caregivers and impose a new income threshold, which remains set at ten times the current CSG value (Coetzee 2013). Under this eligibility estimation, the proportional value of the CSG to caregiver income can, at a minimum, supplement current household income by an additional 10 per cent each month, with the proportional value increasing for lower earning households. In April 2021, the income threshold for single caregivers was R4600 per month (the CSG transfer value was R460), and R9200 joint income per month for married caregivers. Children are age-eligible for this unconditional
³This analysis limits caregivers to mothers, given the nature of this research.
cash transfer from birth, and while initially targeted towards children under the age
of 7, current requirements encompass children up to 18 years old (Mackett 2020).
3.2 Pregnancy Support: Mother’s receiving the CSG while pregnant
While existing literature focuses on the impact of social protection programmes on the recipient (Coetzee 2013, Aguero et al. 2006), the CSG is reported to have spillover effects, resulting in shared benefits for members of recipient households (Coetzee 2013). These spillover effects reinforce the assumption of pooled household income in South Africa. Thus mothers receiving a CSG for their first child may be better able to nourish subsequent children during pregnancy (compared to first children who receive no spillover effects). Hereafter, pregnancy support refers to children who benefit from an older sibling’s CSG while in utero: these children comprise the treated sample. The conceptual design of the pregnancy support effect is illustrated in Figure (1) for additional clarity.
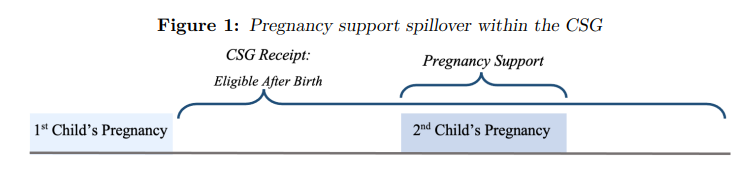
Notes: Figure 1 illustrates a basic timeline, where firstborn children receive (or are eligible to receive but do not) the CSG after birth. Subsequent children may then receive spillover benefits from the CSG while in utero (called pregnancy support).
As pregnancy support can only be received if an older sibling is a CSG beneficiary, firstborn children cannot be eligible for treatment and are excluded from the sample. Additionally, treatment is purely binary since CSG uptake (and therefore pregnancy support) must begin before conception and continue throughout pregnancy or not at all (for non-recipient children). Thus, this paper aims to estimate the benefits of receiving pregnancy support in utero compared to non-recipient children (looking at child health outcomes), where to infer casual analysis, Stable Unit Treatment Value Assumption (SUTVA)4 must hold. Although pregnancy support is encompassed within the CSG, this treatment is redefined from a spillover effect to a pure treatment effect, so that by definition it directly targets non-firstborn children in utero. Under this framework the treatment is now plausibly free of spillover effects; satisfying SUTVA and allowing for causal estimation in further sections.
The following sections use this conceptual framework to examine the existence, magnitude, and persistence of the pregnancy support treatment effect. The hypothesis is that children born into households already receiving treatment will report improved HAZ after birth. Additionally, marginal gains from prenatal support will highlight the importance of intervention during a child’s time in utero.
For SUTVA to hold, child health outcomes must solely depend on the treatment
(pregnancy support), which must be targeted directly towards the child in question
and not the result of a spillover effect from treatments of other individuals.
3.3 The NIDS Data
This paper makes use of rich demographic, welfare and health data from the National Income Dynamic Study (the NIDS). Started in 2008, this survey is the first panel survey to include all household-, individual- and income-level data in South Africa. Data is collected for 28,000 individuals and 7,300 households, who are reinterviewed for successive periods (called ‘waves’) every two years until the fifth wave in 2017 (Woolard & Leibbrandt 2013). Given the non-random sample, the NIDS may suffer from attrition and present biased estimates depending on the research at hand (Ardington & Gasealahwe 2012). All five waves of the NIDS are used to investigate the effect of pregnancy support on HAZ. For each newborn child, the wave of data prior to birth provides context to maternal and household characteristics before conception, while the wave after birth is useful for examining long-term effects and the consequences of lacked pregnancy support.
To examine the impact of treatment while in utero, HAZ is used as a predictor of newborn health. This is a well-established measure of individual health status in the literature, especially among children (Martorell et al. 1994), which acts as proxy for child health status. Z-scores are derived by comparing the child’s height with that of a reference group of well-nourished children known as the WHO Reference 2006 (World Health Organization 2006). Birthweight is also considered as an indicator of health given the association between maternal health and birthweight, as well as birthweight and long-term health implications (Almond & Currie 2011, Hoynes et al.2016, Amarante et al. 2016). In the NIDS data, birthweight is reported by a child’s caregiver, who commonly reports identical birthweights for numerous children. As a result, birthweight is considered for pure average treatment effects but is not included in further estimations
4 The identification issue: treatment and control groups.
The main empirical concern when estimating the pregnancy support effect of the CSG is that of non-random programme placement. The NIDS data reports birth outcomes (HAZ) for children who receive pregnancy support but cannot observe HAZ for the same child as if no treatment occurs. To overcome this missing data is difficult: it is useful to examine the full dataset to establish how the CSG is targeted. Extracts are presented in Table (1): the systematic differences between all treated and untreated households align closely with means testing requirements for the CSG (and thus pregnancy support). Beneficiary households are, on average, poorer, more likely to reside in rural settlements and have less access to running water. African individuals make up 93 per cent of this recipient sample.
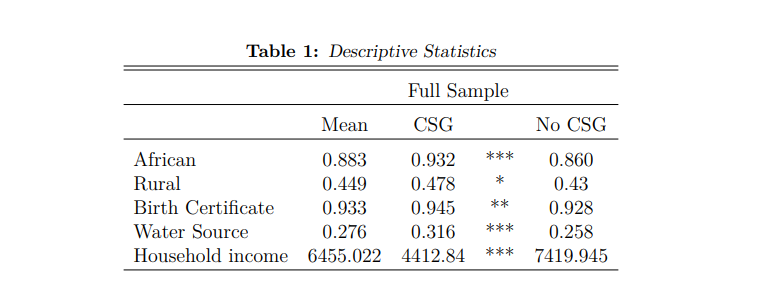
Notes: This table presents descriptive statistics for children and their respective household, who receive a CSG or do not. Only estimates of interest are presented. These calculations make use of the NIDS data and utilise panel weights. Significant differences are starred. * implies a p value<0.10, ** implies a p value<0.05, and *** implies a p value<0.01.
A na¨ıve comparison in the full dataset (Table 1), between children who receive pregnancy support and the remaining untreated sample, would compute biased estimates of the treatment effect because of the large systematic differences between these groups. Given well-defined eligibility criteria for pregnancy support (discussed in Section 3.1), a more appropriate comparison restricts the sample to children who are means-eligible5 to receive treatment, and whose mothers are their primary caregivers (Table 2 makes use of this sample). With means eligibility restrictions in place, the treatment group comprises the group of children who are eligible and receive pregnancy support, while the control group represents errors of exclusion
(the A child who is means-eligible for pregnancy support (treatment) must have an
older sibling who is means and age eligible for the CSG.
6Since the full sample is means eligible to receive the pregnancy support grant,
group of children who are eligible, but do not receive treatment), where the administrative burden7 is listed as the main reason for mothers not applying within the NIDS data (Coetzee 2013). As expected, the control group in Table 2 shares similar observable background characteristics (and is therefore an important potential counterfactual group) to those who are treated because of shared means eligibility status.
The control group (or untreated group) has been used as a counterfactual group in the past (Aguero et al. 2006), with children who are eligible but non-recipients considered to be tardy applicants that merely delay application for the CSG (Coetzee 2013). To account for a mother’s ‘tardiness’, this analysis follows that of Aguero et al. (2006) and Coetzee (2013), introducing a variable which considers a mother’s motivation for grant uptake (labelled eagerness). The inclusion of this control is largely in an attempt to control for unobserved differences between mothers, which could prompt certain mothers to apply (and receive) treatment earlier than others. To compute this control, the difference between the length of time that a mother has been eligible for the CSG and the length of time she has received the CSG (which is zero for the untreated sample) is estimated (Coetzee 2013, Aguero et al. 2006). a child’s caregiver (all of whom are mothers for this analysis) can choose to receive, or not to receive, the CSG (and therefore pregnancy support) for her children. Therefore, treatment status is at the discretion of mothers
The administrative burden refers to caregivers not having the correct documentation. The following most common reason for non-receipt is caregivers reporting that they “have not got around to it yet”.
Under an assumption of constant means eligibility, a mother is eligible to receive the CSG from the birth of her first child. Thus, a smaller estimate is suggestive of earlier grant uptake and a more eager (or motivated) mother
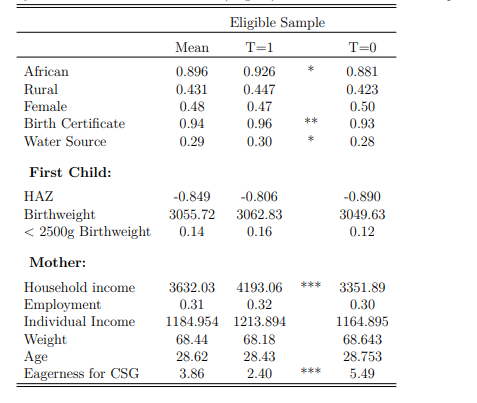
Notes: This table presents descriptive statistics for a sample of children with older siblings who are means eligible for the CSG, and therefore are eligible for pregnancy support themselves. The table is differentiated by treatment status. These calculations make use of the NIDS data and utilise panel weights. Significant differences are starred. * implies a p value<0.10, ** implies a p value<0.05, and *** implies a p value<0.01
The addition of the eagerness control is likely to account for certain unobserved bias that occurs due to different motivations for grant uptake. However, while largely similar characteristics are displayed in Table 2, there are systematic differences which are suggestive of persistent treatment selection biases beyond eligibility criteria: possessing a birth certificate, having easy access to clean water and reporting higher household income make treatment receipt more likely.
A robust analysis of causal impact requires that no treatment sample bias be present so that every eligible child is equally likely to receive pregnancy support. Given the marginal but significant systematic differences between the treatment and control sample, even after controlling for means eligibility for the CSG, additional measures are necessary to ensure accurate treatment effects. Interestingly, first child outcomes are all balanced in Table 2, suggesting that in the absence of the CSG, health outcomes would likely be similar for second born children.
Before moving to the empirical specification, a brief preliminary analysis explores a na¨ıve relationship between pregnancy support status and child health outcomes. The treatment and control groups are taken from Table 2. On average, children who receive pregnancy support have a lower likelihood of being born underweight (less than 2500 grams- see Figure 2A) and improved weight-for-height standardised Z-scores (Figure 2B), compared to those in the control group. Given the reasons outlined above, the treatment and control groups in these figures will not be used
Treatment receipt additionally differs by geographic location. for the empirical estimations in further sections. However, as these groups are used in previous research, they are deemed appropriate for na¨ıve analysis, with the illustrations considered to be suggestive of the true relationship.
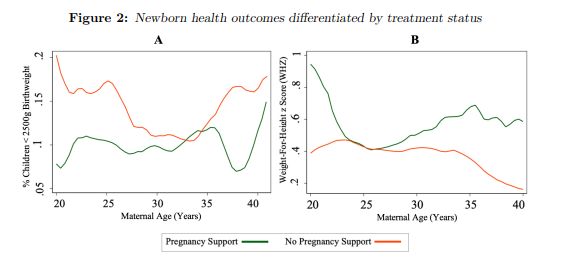
Figure Notes: Local polynomial regressions using the Epanechnikov kernel function are estimated and the graphical results are presented. Two child health outcomes (probability of low birthweight [A] and weight-for-height z-scores [B]) are illustrated, differentiated by treatment status and as a function of maternal age. These naive regressions utilise data from the NIDS Wave 1-5. Panel weights have been applied and 95 per cent confidence intervals are large and not displayed by choice. The sample is limited to the treatment and control group as in Table 2.
5 Empirical Framework
The fact that the CSG is targeted toward poor households and, unlike other wellknown social protection programmes, access is not randomised, leads to challenges in finding a convincing identification strategy for assessing the impact of the programme. Given the non-random sample selection into the CSG and thus into pregnancy support, the previous section restricts the sample to eligible children whose mothers are their primary caregivers. Even after this restriction, there are systematic differences between the treatment and control groups because some mothers receive the grant and others choose not to: this makes it challenging to find an appropriate counterfactual for a pregnancy support recipient.
This paper attempts to account for such treatment sample selection biases by using Augmented Inverse Probability Weighted (AIPW) estimators for cross-sectional and panel data as well as a household Fixed Effects regression. For both specifications, the sample remains limited to children, with mothers as their primary caregivers and who are means eligible for pregnancy support. Additionally, this sample is restricted to African individuals, given their vast proportion of the eligible sample (Eyal & Woolard 2010).
5.1 AIPW Estimators
To control for potential treatment selection bias, this paper uses the Augmented Inverse Probability Weighted (AIPW) estimator to compute an adequate counterfactual group and then estimate the Average Treatment Effect (ATE) of receiving pregnancy support. This method matches the treatment group with untreated individuals who share similar observed characteristics. The AIPW estimator is similar to inverse probability weighting (IPW), with an additional augmentation term to correct for model misspecification.
The AIPW estimator computes the parameters of the treatment model and estimates inverse-probability weights for being included in the treatment sample. Sample restrictions (means eligibility and African individuals) improve the accuracy of matching estimates. The weighted, matched differences and variances between the treatment group and computed counterfactual are presented in Appendix A.1. All mean differences fall below ten per cent and variance differences between 0.5 and 2, leaving minimal risk of systematic differences between the two groups. The AIPW control and treatment groups are thus adequate counterfactuals for one another
Separate regression models are estimated for birth outcomes given the receipt of, or lack of, pregnancy support, resulting in treatment-specific predicted outcomes for each child. A logit model predicts pregnancy support treatment status as a function of maternal age, firstborn child age, province, household water source, wave, and grant uptake eagerness. To maximise the predictive power of this model, factorvariable notation incorporates the quadratic effects of a mother’s age and firstborn child’s age. A linear regression models newborn health outcomes using maternal age, maternal weight, firstborn HAZ, gender, a logged income variable, and household water source as explanatory variables for the outcome.
The weighted means of the treatment-specific predicted outcomes are computed, and the difference between these weighted averages provides the treatment effect estimates, which are reported and discussed in Section 6. In previous literature, failing to account for different treatment intensities has been criticised; however, as discussed in Section 3.2, pregnancy support is plausibly a binary treatment, with children receiving support throughout their time in utero, or none at all.
The benefits of the AIPW estimator are numerous. Robins et al. (1994) created the AIPW estimator by augmenting the Inverse Probability Weighting (IPW) estimator with a weighted average for the outcome model, resulting in reduced variability for the AIPW compared to the IPW and improving estimate efficiency. Given the augmentation term in the model, the AIPW model has a ‘doubly robust’ nature, allowing the estimator to consistently estimate effects as long as one model (the treatment or outcome) is specified correctly1. The AIPW model is flexible compared to other estimators, as it does not require the same covariate specification for both treatment and outcome. In this analysis, treatment uptake hinges on a first child, while the outcome variable considers subsequent children. Thus the flexibility of the AIPW holds vast value for estimation, adding credibility to each model by allowing
Section considers the robustness of this assumption and introduces an alternative treatment specification. However, a binary treatment model is the base model for this paper.
If a propensity score does well in predicting whether a child will receive pregnancy support, then the augmentation term tends to zero in expectation and the model simplifies to the IPW. Contrastingly, for poorly estimated propensity scores, the AIPW model simplifies to a Response Surface Model (RSM) model. different covariate specifications
The AIPW estimator must satisfy an overlap assumption, which ensures the predicted inverse probability weights are within normal range (see Section 7 for discussion, Appendix A.2 for results). Additionally, treatment status is assumed to be independent of potential outcomes after conditioning on observed covariates. If this assumption does not hold, AIPW estimators produce inconsistent treatment effect estimates. In this regard, controlling for a mother’s eagerness to receive treatment assumes that observable variables are able to characterise her behaviour and generate balanced propensity scores. The plausibility of this assumption is discussed in Section 7; however, this assumption cannot be proven. Thus, a household Fixed Effects regression is specified for robustness as the assumption of observable confounders is controlled for at the household level in this model.
5.2 Household Fixed Effects Regression
The specification of this model follows closely that of Sanchez et al. (2020), comparing the marginal differences between the outcomes of younger and older siblings from treated households, with the outcomes of younger and older siblings from nontreated households, in a single period.
Therefore, in this model specification, first children are included in the analysis for comparison to subsequent children. The conceptual design of ‘pregnancy support’ enforces each child receiving treatment to have an older sibling who receives the CSG grant. Similarly, children who lack pregnancy support must have non-CSG recipient Treatment selection bias, potentially problematic in the AIPW model, is controlled for in the household Fixed Effects regression. Latent variables contributing to mothers’ grant uptake are assumed to affect both siblings equally. If this assumption holds, treatment selection bias is removed by controlling for maternal characteristics in the model. The construction of pregnancy support within the CSG ensures this assumption holds: a mother’s motivation to receive the CSG after the birth of her first child also determines her motivation to receive pregnancy support.
The equation below computes the additional impact of receiving pregnancy support for second children compared to receipt of the CSG after birth for first children. The estimation for this model is:
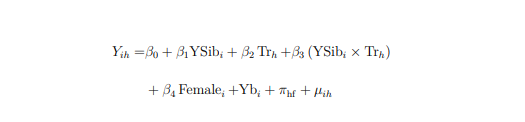
Where Yih is the outcome of child i from household h, observed post-treatment; YSibi takes the value of 1 if child i is the younger sibling and 0 otherwise; Trh takes the value of 1 if household h receives treatment, 0 otherwise; female takes the value of 1 if the child is a female, 0 otherwise; YBirthi is year of birth Fixed Effects; and πhf is a household fixed effect. The coefficient of interest, β3, measures the additional impact of treatment for younger siblings, isolating the pregnancy effect. older siblings
If no difference is estimated, there is no additional gain from receiving support in utero compared to after birth. This estimation considers within-sibling differences at a fixed point in time, with the advantage of requiring no baseline information and removing time heterogeneity.
6 Results
The estimates are reported below by applying the empirical strategies defined in the Section 5. Two key questions are considered in turn, which provide a comprehensive interpretation of the pregnancy support effect:
(i) Is there robust evidence of a pregnancy support treatment effect within the
CSG? If an effect is found, what impact does the treatment have on newborn health
outcomes?
(ii) If receiving pregnancy support affects newborn HAZ, does this effect persist into
childhood, or are non-recipient children able to ‘catch up’?
6.1 The Pregnancy Support Treatment Effect
In Tables (3) and (4), both empirical models are applied, in turn, to answer question (i) robustly.
Table (3) reports the average treatment effect for HAZ as well as an additional This paper has strict page limitations. As a result, all coefficients of non-primary 24 indicator for birthweight. The AIPW model computes an appropriate counterfactual group, using linear models for the outcomes, HAZ and birthweight, and a logit binary treatment regression . All children who are eligible for pregnancy support are pooled together in this model for the advantage of sample size.
On average, non-recipient children weigh 3,073 grams at birth (Table 3), and children receiving pregnancy support benefit from an additional 43.21 grams, which is significant at the 5 per cent level. A significant treatment effect is also estimated for HAZ, where recipients of pregnancy support benefit from an additional 1.76 standard deviations (0.21** HAZ). Notably, while the mean birthweight for untreated children falls comfortably within normal range, the average HAZ is approximately -1.33, considerably close to the WHO defined stunting threshold of -2 (World Health Organization 2006).
interest are not reported. Upon request, full results are available. 13Throughout Section (6), standard errors are robust. 14Recall that the conceptual design of pregnancy support restricts firstborn child from eligibility because they cannot have an older sibling who receives the CSG during their time in utero.
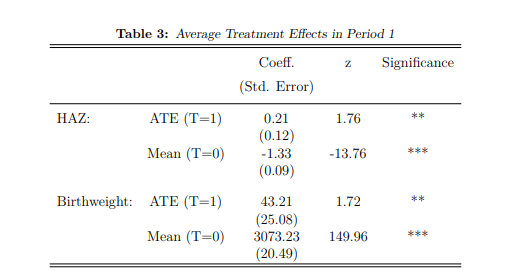
Notes: Table 3 uses AIPW estimators to compute the mean estimates for two child health outcomes (birthweight and HAZ) for children who do not receive pregnancy support while in utero (T=0). The Average Treatment Effect (ATE) reports the additional gain for children who receive pregnancy support (T=1) in comparison to the mean estimates. Estimated coefficients as well as standard deviations are reported, and significant results are starred: * implies a p value<0.10, ** implies a p value<0.05, and *** implies a p value<0.01.
In Table (4), the household Fixed Effects regression is applied and resulting HAZ estimates are presented. This model compares paired-siblings within a single period (at different ages), making HAZ an appropriate outcome as it is standardised across age. Birthweight is excluded due to data issues discussed in Section (3.3). The advantage of Table (4) estimates (compared to the AIPW estimator in Table 3) is that fixed household and child characteristics related inter alia to selection into treatment are controlled.
The estimates in Table (4) suggest that receiving treatment in utero holds additional gains compared to receipt of the CSG only after birth. This result is statistically significant, with younger siblings reporting an increased HAZ of 0.4 because of early treatment occurring while in utero. The ‘exposure’ estimate is an additional control introduced to this model, and defined as the percentage of a firstborn child’s life during which he/she received the CSG15. Exposure has the opposite sign to the treatment effect, the result of high correlation between the impact of pregnancy support receipt and firstborn exposure to the CSG.
Exposure (%) is calculated using the data on the month and year in which first children were born as well as the responses from mothers regarding the initial date of receipt of the CSG. For this analysis, nine months are added to each firstborn child’s age, signifying time in utero (where no grant was received).
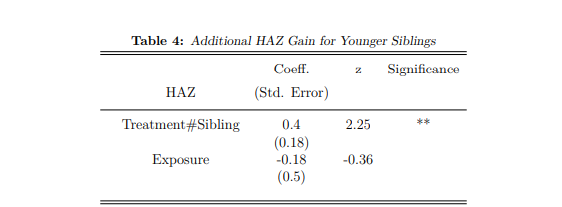
Notes: Table 4 uses a household Fixed Effects regression model to estimate the additional gain a younger sibling receives from treatment while in utero, compared to older siblings who receive the CSG after birth. The treatment effect (Treatment#Sibling) is thus able to isolate the marginal gains from intervention during pregnancy. The estimated coefficients and standard deviations are reported, and significant results are starred: * implies a p value<0.10, ** implies a p value<0.05, and *** implies a p value<0.01
Tables (3) and (4) estimate the same treatment effect, with the latter reporting a larger gain in HAZ (0.4 compared to 0.21). If first children have delayed CSG uptake, the increased treatment effect in Table 4 is plausibly due additional gains for children receiving support in utero, compared to first children receiving the CSG many months after birth (rather than from birth). These results collectively provide evidence for the pregnancy support effect within the CSG and suggest that receiving treatment while in utero results in positive gains for HAZ.
6.2 The persistence of treatment effects.
The results thus far have shown that the pregnancy support effect has an underlying impact within the CSG, and that children who receive support in utero have substantially improved HAZ. From a policy perspective it is important to investigate the persistence of this effect. If a child who does not receive pregnancy support, but receives the CSG after birth, can fully catch up in HAZ, then targeting pregnant mothers is a waste of resources. For this estimation, an additional post-treatment period is introduced (period 2), and the sample is restricted (while maintaining previous restrictions) to CSG beneficiaries. It is important to note that this model only considers the base sample of second children: Therefore, CSG receipt refers to CSG uptake for second children when they become eligible (after birth). This is independent of the same child’s pregnancy support treatment status (because pregnancy support hinges on CSG receipt of the first child).
The premise of catch up growth offers the potential to minimise negative effects by offering interventions to improve outcomes. Prior to estimation, two long-term effects are hypothesised.
(i) A post-birth CSG of equal value is given to both treatment groups. If proportional effects exist, a grant of equal value will have proportionately larger returns for the group with initially lower HAZ scores. In this case, the untreated sample receives additional gains in HAZ through CSG income transfers. This additional gain is unlikely to be large enough to offset the lack of prenatal support.
(ii) Given the persistence of inter-generational nutrition and poverty, children with poor health may need larger income transfers to see significant marginal gains. Under this hypothesis, a child receives exponential gains in HAZ using period one for base estimates. Therefore, children with initially poor health cannot gain as much additional value from the grant income as healthier children are able to. One possible explanation for this is that children with better health can spend the grant’s total value on preventative rather than curative care. Children with low HAZ scores then bear an initially higher economic burden, spending most of their grant income on curative care. Under this hypothesis, healthier children benefit from higher returns to health.
Table (5) reports the marginal gains from CSG receipt for both treatment groups, applying an outcome differenced estimation to the AIPW model16. The results are indicative of hypothesis (i): untreated children report more considerable marginal gains (0.24 increase in HAZ) compared to the treated sample (-0.13 smaller increase in HAZ). This positive result suggests that children who did not receive support while in utero can significantly improve their HAZ scores during early childhood, given CSG receipt. However, this additional gain is insufficient to compensate for the lack of prenatal treatment. The catch-up growth of 0.24 HAZ in Table (5) marginally surpasses the improved HAZ of 0.21 that children who received pregnancy support
This expansion of the AIPW is simple, changing only the outcome variable. HAZ (which was estimated in period 1 and then 2) is replaced with the differenced variable (period 2 HAZ – period 1 HAZ).reported in period 1 (Table 3). After the treated group receives the CSG, their HAZ is significantly improved in comparison.
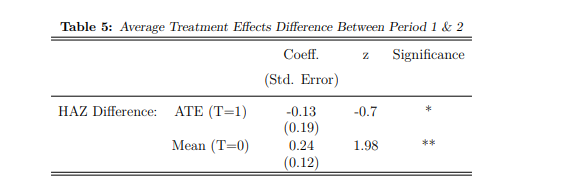
Notes: Table 5 makes use of AIPW estimators to report the mean change in HAZ from period 1 to period 2 (given additional cash transfers from the CSG) for children who did not receive pregnancy support in utero (T=0). The Average Treatment Effect reports the marginal difference for the treated group (T=1) compared to the mean (T=0). Estimated coefficients as well as standard deviations are reported, and significant results are starred: * implies a p value<0.10, ** implies a p value<0.05, and *** implies a p value<0.01.
These results emphasise the importance of prenatal intervention for child health. A lack of support for children while in utero undermines existing resources aimed at children after birth, limiting the CSG from being fully efficient.
7 Robustness checks and potential expansions
The importance of a suitable counterfactual group is imperative to the validity of observational studies. Given the difficulty of overcoming treatment selection biases which are shown to persist beyond eligibility requirements, two different models (AIPW estimator and household Fixed Effects regression) are applied for robust analysis. Using these models, the existence, magnitude and persistence of the pregnancy support effect are estimated. The corroboration of these effects and varying model specifications advantage the credibility of the findings in this paper.
The overlap condition, where every child has a positive nonzero probability of receiving pregnancy support, is reported in Appendix A.1. Because the AIPW estimator weights observations in accordance with their observed similarity, the propensity score distributions do not need to be perfectly corresponding, but sufficient overlap is important. The overlap assumption is satisfied, as are the covariate balance tests (refer to Section 5.1).
The strictest assumption under the AIPW model is that the conditional independence assumption holds for the estimated propensity scores. Given the importance of this assumption for the credibility of results, this paper extensively explores treatment selection biases and how to compute an adequate counterfactual group (see Sections 4 and 5). Restricting samples to means eligible recipients and African individuals largely improves the accuracy of matching methods, with certain literature using this control as the counterfactual. In this paper, additional matching criteria are applied through the AIPW estimator: the inclusion of an observed eagerness control, which proxies for a mother’s eagerness for child support grant uptake (which is unobserved in the data), increases the likelihood that the model is accurately specified. However, baseline information would provide an additional measure of robustness and remove any potential for unobserved heterogeneity. For this reason, the household Fixed Effects regression is estimated, examining siblings of varying ages within a single period. This model plausibly removes treatment selection bias (discussed in Section 5) by controlling for latent maternal characteristics and provides the advantage of not requiring bassline information. Since the estimated results for both models corroborate one another (in direction and with similar magnitudes), this suggests that the AIPW is correctly specified, with the household Fixed Effects estimation considered to hold additionally robust (given no untestable assumptions) results.
As treatment occurs during pregnancy, no baseline is logical. Although the household Fixed Effects model computes robust estimates without a baseline, the nature of a single time period omits birthweight from further exploration. Therefore, as a robustness test for the AIPW estimated birthweight treatment effects, this paper explores an additional estimation which imputes a baseline, using the observation from the firstborn sibling at baseline to represent what would have been the baseline for the younger sibling in the absence of the programme. Sanchez et al. (2020) use this approach to account for similar constraints, allowing variation across time but not age. Given the age invariance of birthweight, it is an appropriate outcome for this model; however, the computed estimate is positive but insignificant.
Until now, treatment has referred to a child’s time in utero; therefore, the receipt has been binary to indicate the entire pregnancy period or none. In the household Fixed Effects estimation, the effect of treatment receipt and exposure proves challenging to disentangle, but is suggestive of a relationship. The literature suggests the effectiveness of targeting pregnant women as opposed to later intervention is interlinked to the persistence of foetal health effects (Hoddinott et al. 2008, Victora et al. 2008). Thus, given the relationship between maternal and child health, the number of grants a mother receives or the length of her exposure to treatment may bear additional consequences for the inter-generational transmission of nutrition
Table 3 is thus re-estimated to include the duration of maternal grant receipt and the number of grants received before conception (see Appendix A.3). Model specifications remain unchanged given the flexible nature of AIPW that allows for categorical treatment variables. The estimates are minor and do not suggest significance. However, the additional treatment groups make matching a counterfactual group more complex; thus, these results are obtained from unbalanced samples.
In Section 6.2, the persistence of the pregnancy support effect is explored, exploiting additional income from the CSG after birth to determine catch-up potential. These results belie that some children have more exposure to the CSG than others; thus, Table 5 estimates are determined using the average exposure. Disentangling the effect between exposure and uptake of CSG is difficult but important: the estimates in table 5 report that untreated children cannot fully catch up with HAZ through CSG receipt of an average exposure time. If an earlier uptake of the CSG, and thus greater exposure, is able to improve the marginal gains for the untreated group, this is equally important for policy. A brief overview is provided in Appendix A.4, presenting the results and an explanation of the sample size versus accuracy tradeoff. The most improved HAZ for non-recipient children occurs when CSG uptake begins at young ages for all children (high exposure) – although full catch-up is not achieved.
The general findings of this paper support the underlying assumption of pooled household income. Under this assumption, the CSG benefits household members similarly to beneficiaries. If this assumption is slightly relaxed, the intended beneficiary may receive more benefits from the CSG than household members receiving spillover effects. Thus, a grant given directly to pregnant mothers may have a considerably larger impact than the results in this paper. However, this paper does not attempt empirical estimations to this effect.
8 Conclusion and Policy Insights
In this paper, the AIPW estimator and household Fixed Effects regression model are used to prove the existence of a pregnancy support spillover effect robustly. Mothers receiving a CSG for their firstborn child give birth to second children with improved outcomes (compared to second children without additional support). This effect is large and persistent, with children who lacked support in utero still bearing this burden in the following periods, even after receiving additional monthly income from the CSG from very young ages.
These results hold important implications. Current studies assessing the CSG in South Africa omit this effect, likely underestimating the impact of the CSG by precluding the pregnancy period. Simply appending this period to existing literature would violate the SUTVA assumption, given that pregnancy support is a spillover from another individual, different to the CSG recipient under examination. Instead, future research should pool household CSG income (as in this paper), overcoming this causal analysis violation and more comprehensively estimating the CSG impact. The inclusion of this effect within CSG impact evaluation literature is critical. When including the pregnancy period, this paper shows even greater health inadequacies for children residing in non-CSG-recipient households. As South Africa suffers from one of the highest rates of inequality in the world, it is fundamentally important to understand the root causes of how inequality is perpetuated throughout generations
Support for mothers during pregnancy is increasingly recognised as important for a child’s development, leading to growing support for pregnancy grants (Chersich et al., 2016). The large and significant effects of the pregnancy grant when evaluated within the CSG and the lack of catch-up potential without pregnancy support provide justification for the CSG being extended explicitly into pregnancy. An expansion into this period would directly target prenatal health, which may have even more significant impacts than the estimates found in this paper (which only considers spillover effects). Interventions that target pregnant women are more effective than later, curative interventions due to the persistence and long-term impacts on foetal health.
This paper does not consider the feasibility or practicality of extending the CSG into pregnancy; however, Cherish et al. (2016) suggest that this possibility exists. Additional research should also consider fertility trends given an expansion (although for the existing CSG this is not problematic), and the potential impact from targeting mothers even before the conception of children.
The South African CSG has long been recognised for its effectiveness in supporting early childhood development and ultimately alleviating poverty and inequality (Aguero et al. 2006). However, the CSG currently ignores a fundamental part of a child’s development: the 270 days spent in utero. By applying an AIPW estimator and household Fixed Effects regression model, this paper demonstrated the strong potential that a support grant received during pregnancy could have for child development outcomes. The findings suggest that extending the Child Support Grant to support pregnant mothers could be a powerful means to reduce the persistently high rates of poverty and inequality plaguing South African society.
The Robustness section of this paper provides a brief analysis of mothers who receive more grants (quantity) or have a longer exposure (duration) before conception,
finding no significant results. Future research should explore this further.
References
Aguero, J., Carter, M. & Woolard, I. (2006), ‘The impact of unconditional cash
transfers on nutrition: The south african child support grant’.
Almond, D. & Currie, J. (2011), ‘Killing me softly: The fetal origins hypothesis’,
Journal of economic perspectives 25(3), 153–72.
Amarante, V., Manacorda, M., Miguel, E. & Vigorito, A. (2016), ‘Do cash transfers
improve birth outcomes? evidence from matched vital statistics, program, and
social security data’, American Economic Journal: Economic Policy 8(2), 1–43.
Andersen, S. L. (2003), ‘Trajectories of brain development: point of vulnerability or
window of opportunity?’, Neuroscience & Biobehavioral Reviews 27(1-2), 3–18.
Ardington, C. & Gasealahwe, B. (2012), ‘Health: analysis of the nids wave 1 and 2
datasets’.
Barker, D. J. (1990), ‘The fetal and infant origins of adult disease.’, BMJ: British
Medical Journal 301(6761), 1111.
Black, R. E., Victora, C. G., Walker, S. P., Bhutta, Z. A., Christian, P., De Onis,
M., Ezzati, M., Grantham-McGregor, S., Katz, J., Martorell, R. et al. (2013),
‘Maternal and child undernutrition and overweight in low-income and middleincome countries’, The lancet 382(9890), 427–451.
Casale, D. (2020), ‘Recovery from stunting in early childhood and subsequent school38
ing outcomes: Evidence from nids waves 1–5’, Development Southern Africa 37(3), 483–500.
Casale, D., Desmond, C. & Richter, L. (2014), ‘The association between stunting
and psychosocial development among preschool children: a study using the s
outh a frican b irth to t wenty cohort data’, Child: care, health and development 40(6), 900–910.
Caulfield, L. E., de Onis, M., Bl¨ossner, M. & Black, R. E. (2004), ‘Undernutrition as
an underlying cause of child deaths associated with diarrhea, pneumonia, malaria,
and measles’, The American journal of clinical nutrition 80(1), 193–198.
Chang, G., Favara, M. & Novella, R. (2022), ‘The origins of cognitive skills and
non-cognitive skills: The long-term effect of in-utero rainfall shocks in india’,
Economics & Human Biology 44, 101089.
Chersich, M., Luchters, S., Blaauw, D., Scorgie, F., Kern, E., Van den Heever, A.,
Rees, H., Peach, E., Kharadi, S. & Fonn, S. (2016), ‘Safeguarding maternal and
child health in south africa by starting the child support grant before birth: Design lessons from pregnancy support programmes in 27 countries’, South African
Medical Journal 106(12), 1192–1210.
Coetzee, M. (2013), ‘Finding the benefits: Estimating the impact of the s outh a
frican child support grant’, South African Journal of Economics 81(3), 427–450.
Danaei, G., Andrews, K. G., Sudfeld, C. R., Fink, G., McCoy, D. C., Peet, E., 39
Sania, A., Smith Fawzi, M. C., Ezzati, M. & Fawzi, W. W. (2016), ‘Risk factors
for childhood stunting in 137 developing countries: a comparative risk assessment
analysis at global, regional, and country levels’, PLoS medicine 13(11), e1002164.
de Rooij, S. R., Wouters, H., Yonker, J. E., Painter, R. C. & Roseboom, T. J. (2010),
‘Prenatal undernutrition and cognitive function in late adulthood’, Proceedings of
the National Academy of Sciences 107(39), 16881–16886.
Devereux, S., Jonah, C. & May, J. (2019), ‘How many malnourished children are
there in south africa? what can be done’, Putting Children First: New frontiers in
the fight against poverty in Africa CROP International Poverty Studies 7, 157–86.
Dewey, K. G. & Begum, K. (2011), ‘Long-term consequences of stunting in early
life’, Maternal & child nutrition 7, 5–18.
Duflo, E. (2003), ‘Grandmothers and granddaughters: old-age pensions and intrahousehold allocation in south africa’, The World Bank Economic Review 17(1), 1–25.
Eyal, K. & Woolard, I. (2010), ‘Female labour force participation and the child
support grant in south africa’.
Gonz´alez, L. & Trommlerov´a, S. (2022), ‘Cash transfers before pregnancy and infant
health’, Journal of Health Economics 83, 102622.
Grow Great (2021), ‘Investigating hunger and mental health among pregnant women
in the cape metro area during the 2020 covid-19 pandemic’, CoCare Maternal Support Study .
Hirano, K. & Imbens, G. W. (2004), ‘The propensity score with continuous treatments’, Applied Bayesian modeling and causal inference from incomplete-data perspectives 226164, 73–84.
Hoddinott, J., Maluccio, J. A., Behrman, J. R., Flores, R. & Martorell, R. (2008),
‘Effect of a nutrition intervention during early childhood on economic productivity
in guatemalan adults’, The lancet 371(9610), 411–416.
Hoynes, H., Schanzenbach, D. W. & Almond, D. (2016), ‘Long-run impacts of childhood access to the safety net’, American Economic Review 106(4), 903–34.
Katepa-Bwalya, M., Mukonka, V., Kankasa, C., Masaninga, F., Babaniyi, O. &
Siziya, S. (2015), ‘Infants and young children feeding practices and nutritional
status in two districts of zambia’, International breastfeeding journal 10(1), 1–8.
Mackett, O. (2020), ‘Social grants as a tool for poverty reduction in south africa? a
longitudinal analysis using the nids survey.’, African studies quarterly 19(1).
Martorell, R., Khan, L. K. & Schroeder, D. G. (1994), ‘Reversibility of stunting:
epidemiological findings in children from developing countries.’, European journal
of clinical nutrition 48, S45–57.
May, J. & Timaeus, I. M. (2014), ‘Inequities in under-five child nutritional status
in south africa: what progress has been made?’, Development Southern Africa 31(6), 761–774.
Norman, R., Bradshaw, D., Schneider, M., Joubert, J., Groenewald, P., Lewin, S.,
Steyn, K., Vos, T., Laubscher, R., Nannan, N. et al. (2007), ‘A comparative risk
assessment for south africa in 2000: towards promoting health and preventing
disease’, South African Medical Journal 97(8), 637–641.
Paxson, C. & Schady, N. (2005), ‘Child health and economic crisis in peru’, The
World bank economic review 19(2), 203–223.
Qadir, M. & Bhutta, Z. A. (2009), Low birth weight in developing countries, in
‘Small for Gestational Age’, Vol. 13, Karger Publishers, pp. 148–162.
Sanchez, A., Melendez, G. & Behrman, J. (2020), ‘The impact of the juntos conditional cash transfer programme in peru on nutritional and cognitive outcomes:
does the age of exposure matter?’.
Shonkoff, J. P., Garner, A. S., on Psychosocial Aspects of Child, C., Family Health,
Committee on Early Childhood, A., Care, D., on Developmental, S., Pediatrics,
B., Siegel, B. S., Dobbins, M. I., Earls, M. F., Garner, A. S., McGuinn, L., Pascoe,
J. & Wood, D. L. (2012), ‘The lifelong effects of early childhood adversity and
toxic stress’, Pediatrics 129(1), e232–e246.
Thompson, R. A. & Nelson, C. A. (2001), ‘Developmental science and the media:
Early brain development.’, American Psychologist 56(1), 5.
UNICEF (2012), ‘The south african child support grant impact assessment: Evidence from a survey of children, adolescents and their households’.
Victora, C. G., Adair, L., Fall, C., Hallal, P. C., Martorell, R., Richter, L., Sachdev,
H. S., Maternal, Group, C. U. S. et al. (2008), ‘Maternal and child undernutrition:
consequences for adult health and human capital’, The lancet 371(9609), 340–357.
Waidler, J. & Devereux, S. (2019), ‘Social grants, remittances, and food security:
does the source of income matter?’, Food Security 11(3), 679–702.
Woolard, I. & Leibbrandt, M. (2013), ‘The evolution and impact of unconditional
cash transfers in south africa’, Development Challenges in a Postcrisis World p. 363.
World Bank, G. I. . C. T. D. (2006), 2006 Information and communications for
development: Global trends and policies, World Bank Publications.
World Health Organization (2006), WHO child growth standards: length/height-forage, weight-for-age, weight- for-length, weight-for-height and body mass index-forage: methods and development, World Health Organization.
World Health Organization (2015), Trends in maternal mortality: 1990-2015: estimates from WHO, UNICEF, UNFPA, World Bank Group and the United Nations
Population Division, World Health Organization.
Zembe-Mkabile, W., Ramokolo, V., Sanders, D., Jackson, D. & Doherty, T. (2016),
‘ The dynamic relationship between cash transfers and child health: can the child
support grant in south africa make a difference to child nutrition?’, Public health
nutrition 19(2), 356–362.
A Appendix
A.1 Covariate Balance Tests AIPW
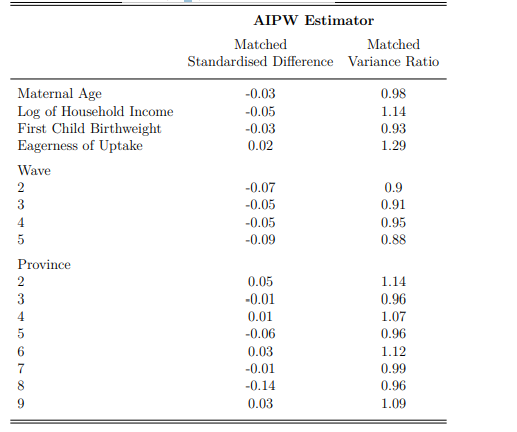
Notes: This is an extract from the AIPW logit estimation of the treatment and counterfactual group. The sample was restricted to means eligible African children before matching techniques were applied. All standardised differences and variance ratios are within satisfactory ranges for the matched samples; thus, no problematic systematic differences are suggested.
A.2 The Overlap Assumption
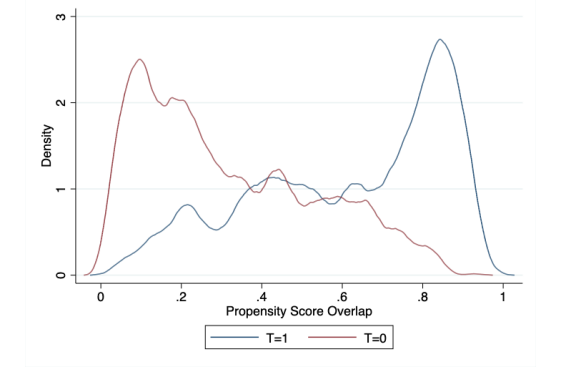
Notes: This graph depicts the propensity score overlap for the AIPW estimator model and has a sufficient overlap of scores for the assumption to be satisfied. (T=1) and (T=0) suggest treatment status as defined in the main text of this paper
A.3 Non-binary Treatment Effects

Notes: This table uses an AIPW estimator to compute the mean estimate for child HAZ using a categorical treatment variable, where the categories indicate a range of quantities of CSGs received by mothers before conception. Given data limitations, there are only two categories (high quantity and low quantity). This specification restricts non-recipient children from the model. The mean HAZ is reported for the treatment group (1), while the ATE shows additional gain in HAZ for children in the treatment group (2) (whose mothers receive a greater quantity of CSG). Estimated coefficients and standard deviations are reported, and significant results are starred: * implies a p value<0.10, ** implies a p value<0.05, and *** implies a p value<0.01.

Notes: Using a categorical treatment variable, this table uses an AIPW estimator to compute the mean estimate for child HAZ. The categories indicate the duration of CSG receipt for mothers before conception (separated into three groups). This specification restricts non-recipient children from the model. The mean HAZ is reported for the treatment group (1), whose mothers have received the CSG for the shortest duration, while the ATE shows additional gain in HAZ for children in treatment groups (2) and (3) (whose mothers have received the CSG for longer periods). Estimated coefficients and standard deviations are reported, and significant results are starred: * implies a p value<0.10, ** implies a p value<0.05, and *** implies a p value<0.01.
A.4 Varying Exposure Thresholds
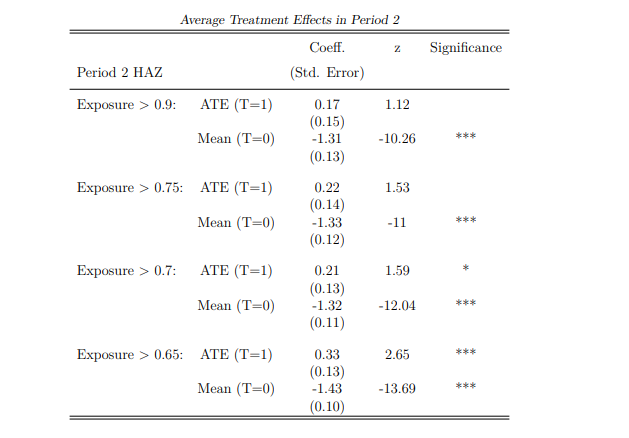
Notes: This table uses AIPW estimators in period 2 to compute the mean estimates for child HAZ for children who do not receive pregnancy support in utero (T=0). The Average Treatment Effect (ATE) reports the additional gain for children who receive pregnancy support (T=1) compared to mean estimates. In this table, subsample analysis accounts for non-homogeneous uptake of treatment, resulting in differing exposure periods. Children who receive the CSG at young ages are exposed for a larger proportion of their life (indicated by a higher exposure %). The first sub-sample considers children exposed to the CSG for nearly all their life (more than 90%): this threshold lowers throughout sub-samples. With high exposure, CSG receipt is more homogeneous, making the results more accurate but decreasing the sample size. Estimated coefficients and standard deviations are reported, and significant results are starred: * implies a p value<0.10, ** implies a p value<0.05, and *** implies a p value<0.01
