- Images below confirm clinical responses seen in patients with metastatic orbital (eye), temporal (brain), liver, and spine lesions
- No Bria-IMT™ related discontinuations reported to date
- Bria-IMT regimen continues under Fast Track Designation from US FDA
PHILADELPHIA and VANCOUVER, British Columbia, Jan. 28, 2026 (GLOBE NEWSWIRE) — BriaCell Therapeutics Corp. (Nasdaq: BCTX, BCTXW, BCTXZ, BCTXL) (TSX: BCT) (“BriaCell” or the “Company”), a clinical-stage biotechnology company developing novel immunotherapies to transform cancer care, releases new images highlighting resolution of metastatic breast cancer lesions in patients with orbital (eye), temporal lobe (brain), liver, and spinal involvement. Survival details on these and other select patients in its Phase 2 study, along with comparable populations, were previously reported.
| Table 1: Select Patients | ||||
| Patient/Subtype | Months Survival | Age | Number of Prior Regimens |
Cycles of Bria-IMT |
| 11-018/ER+/PR+/HER2+ (Example 1 below) |
27 | 66 | 8; including ENHERTU | 35 |
| 15-005/ER+/PR+/HER2- (Example 2 below) |
27 | 44 | 5 | 6 |
| 15-006/ER+/PR-/HER2- (Example 3 below) |
25 | 64 | 8; including TRODELVY | 4 |
Note that Trodelvy and Enhertu are antibody-drug conjugates recently approved for late-stage breast cancer.
“The CD8 ImmunoPET images are remarkable, verifying the ability of the BriaCell treatment to activate CD8+ cytotoxic (“killer”) T cells and induce their infiltration into the cancerous tumors. This is key to the mechanism of action of the BriaCell approach and likely play a role in enhancing the long-term survival of patients even after they come off study,” stated Dr. William V. Williams, BriaCell’s President and CEO.
Example 1: Patient 11-018
A 66-year-old woman with ER+/PR+/HER2+ metastatic breast cancer, heavily pretreated with 8 prior lines of therapy, including an antibody-drug conjugate (Enhertu), remains alive 27 months post-enrollment. At baseline, she presented with metastatic involvement of the right orbit (behind the eye), right temporal lobe of the brain and multiple skeletal sites. Following treatment, she achieved complete resolution of the temporal lobe metastasis, substantial improvement in the orbital lesion and stable disease in the bone. She remained on study for 26 months after initiating treatment and receiving 35 cycles of therapy. Images though 20 months for this patient have been previously described (link). Shown here are updated images through 2 years with measurements superimposed (through 18 months for the temporal lobe brain metastases).
Example 1 Images (Patient 11-018): Bria-IMT treatment resulted in complete resolution of the right temporal lobe lesion and continued regression of the right orbital (behind the eye) tumor. Measurements of lesion sizes are shown.
LA = long axis. SA = short axis.
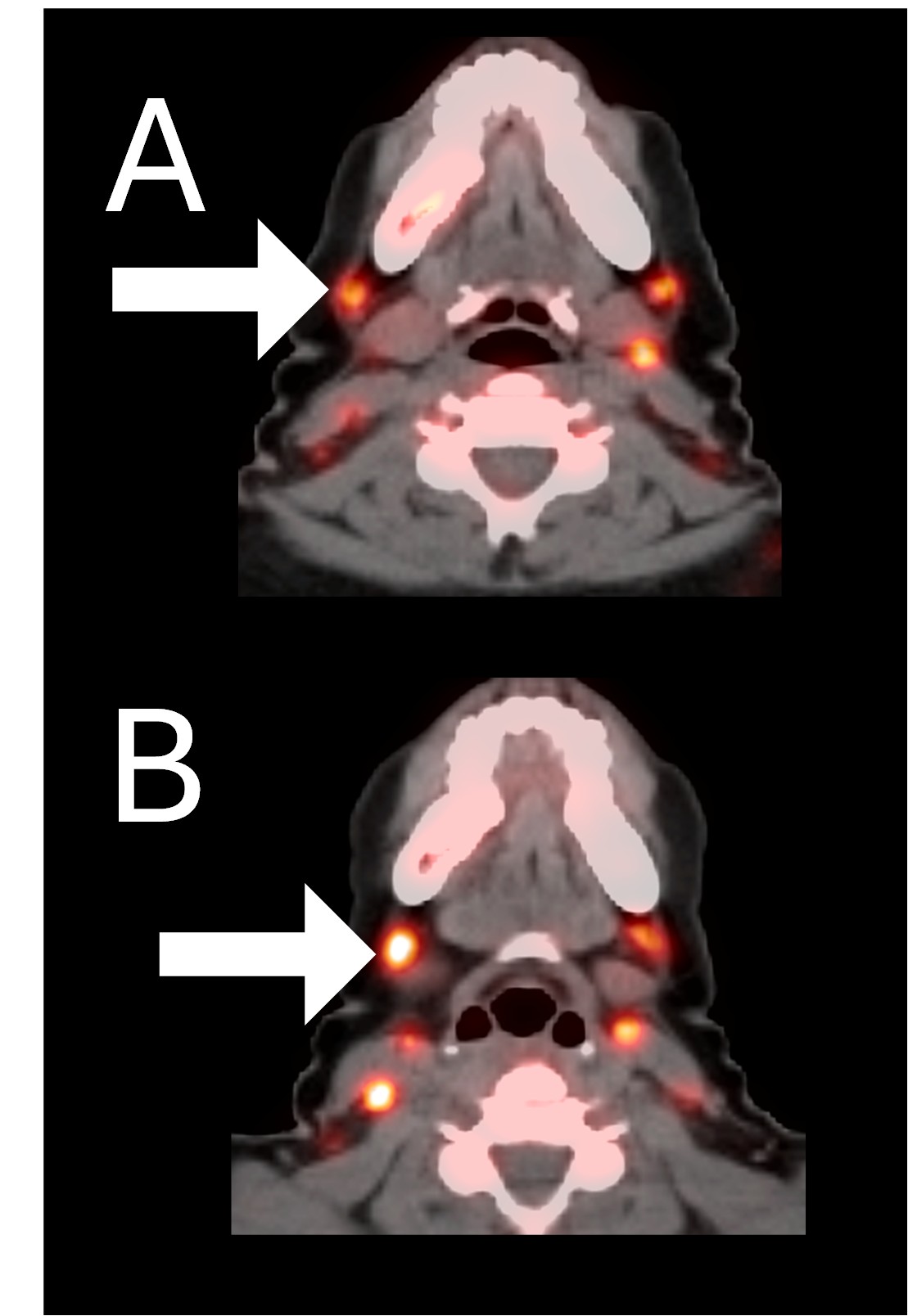
Example 2: Patient 15-005:
A 44-year-old woman with ER+/PR+/HER2- metastatic breast cancer, previously treated with 5 prior lines of therapy. At baseline, she presented with metastases to the spine. She completed 6 cycles of therapy achieving stable disease as her best response and remains in survival follow-up 27 months after study entry.
Before BriaCell Treatment Image A: CD8 ImmunoPET image
Pre-treatment imaging of cervical (neck) lymph nodes with moderate uptake indicating presence of some CD8+ cytotoxic (“killer”) T cells.
After BriaCell Treatment Image B: CD8 ImmunoPET image
Post treatment enhancement of cervical (neck) lymph nodes indicating immune system activation and increased presence of CD8+ cytotoxic T cells.
Example 2 Images (Patient 15-005): CD8 ImmunoPET images pre (A) and post (B) Bria-IMT treatment
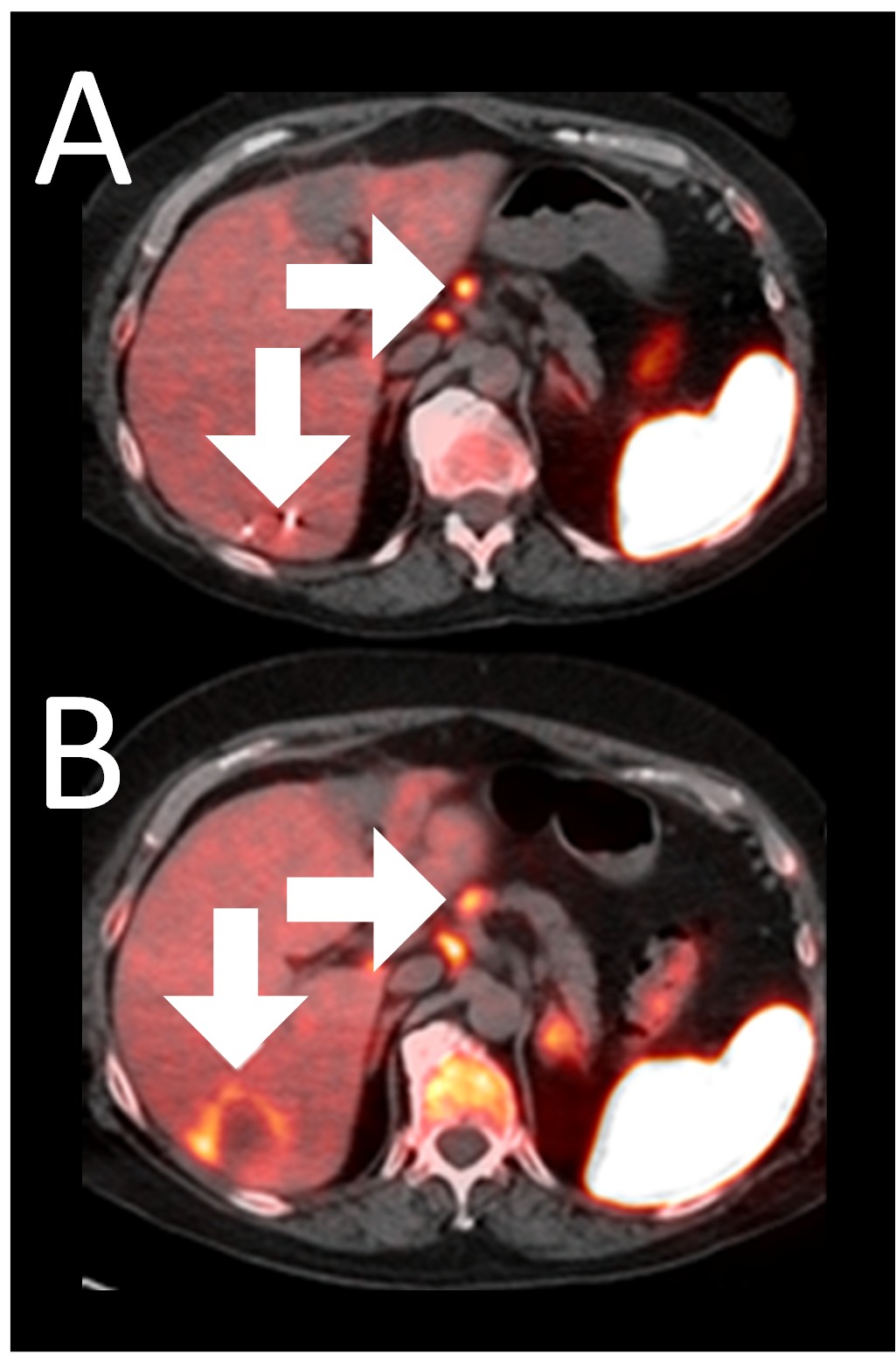
Example 3: Patient 15-006:
A 64-year-old woman with ER+/PR-/HER2- metastatic breast cancer, heavily pre-treated with 8 prior lines of therapy, including the antibody-drug conjugate Trodelvy, remains alive 25 months post-enrollment. At baseline, presented with hepatic metastasis.
Before BriaCell Treatment Image A: A liver metastasis (lower arrow) is “cold,” indicating minimal to no CD8+ cytotoxic T cells in the tumor while enlarged lymph nodes (upper arrow) show moderate uptake.
After BriaCell Treatment Image B: Swelling (induration) around the metastasis (lower arrow) demonstrates the liver metastasis has become “hot”, indicating marked CD8+ cytotoxic T cell infiltration while further lymph node enlargement is consistent with increased activity (upper arrow) indicating increased CD8+ T cells.
Example 3 Images (Patient 15-006): Combined MRI and CD8 ImmunoPET images Pre (A) and Post (B) Bria-IMT treatment
The Phase 2 study enrolled 54 heavily pre-treated metastatic breast cancer patients (median six prior therapies) who received the Bria-IMT regimen plus a checkpoint inhibitor. Of these, 37 patients were treated with the same formulation now being evaluated in the pivotal Phase 3 study (NCT06072612). Significantly, no Bria-IMT related discontinuations have been reported to date.
About BriaCell Therapeutics Corp.
BriaCell is a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care. More information is available at https://briacell.com/.
Safe Harbor
This press release contains “forward-looking statements” that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release may be identified by the use of words such as “anticipate,” “believe,” “contemplate,” “could,” “estimate,” “expect,” “intend,” “seek,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “target,” “aim,” “should,” “will,” “would,” or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements, including those regarding BriaCell’s interpretation of patient imaging results demonstrating tumor regression and immune activation, and the potential implications of these findings for the ongoing Phase 2 and pivotal Phase 3 studies, are based on BriaCell’s current expectations and are subject to inherent uncertainties, risks, and assumptions that are difficult to predict. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully under the heading “Risks and Uncertainties” in the Company’s most recent Management’s Discussion and Analysis, under the heading “Risk Factors” in the Company’s most recent Annual Information Form, and under “Risks and Uncertainties” in the Company’s other filings with the Canadian securities regulatory authorities and the U.S. Securities and Exchange Commission, all of which are available under the Company’s profiles on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov. Forward-looking statements contained in this announcement are made as of this date, and BriaCell Therapeutics Corp. undertakes no duty to update such information except as required under applicable law.
Neither the Toronto Stock Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Toronto Stock Exchange) accepts responsibility for the adequacy or accuracy of this release.
Contact Information
Company Contact:
William V. Williams, MD
President & CEO
1-888-485-6340
[email protected]
Investor Relations Contact:
[email protected]
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/f8e56823-b47a-4a4e-b05d-50190c9b5aab
https://www.globenewswire.com/NewsRoom/AttachmentNg/4a0770cf-c64f-46f1-b740-7ac7d63a49c0
https://www.globenewswire.com/NewsRoom/AttachmentNg/7579c49e-8844-4b30-9939-183ce817ca73

