FREEHOLD, N.J., Dec. 18, 2024 (GLOBE NEWSWIRE) — Channel Therapeutics Corporation, (“Channel” or the “Company”), (NYSE American: CHRO), a pioneer in the development of non-opioid pain treatment therapeutics, today announced that it achieved its endpoints in two pre-clinical in vivo models of the Company’s nerve block formulations for acute pain, showing material improvement over the existing standard of care, bupivacaine, in both efficacy and duration.
“We are very pleased with the results, which potentially demonstrate that nerve blocks with our NaV1.7 inhibitors may be viable options for the treatment of acute and postoperative pain,” stated Dr. Eric Lang, Chief Medical Officer of Channel. “Additionally, we believe this drug has the potential to improve on existing postoperative therapeutic options while opening the door for success with our other programs,” concluded Dr. Lang.
About the trial
The Company performed a thermal hyperalgesia test in rodents with a placebo arm, bupivacaine arm and four arms of the main formulations of the Company’s molecule. The Company also performed a mechanical allodynia test in rodents with the same arms as above. For both models, the drugs were administered as a sciatic nerve block. All four Company formulations showed a depot effect in excess of four days, an improvement over bupivacaine, the current standard of care.
Results
The results of the thermal hyperalgesia results are shown in the chart below. After thirty minutes, three of the four formulations showed materially better efficacy than bupivacaine, with each of the three being statistically superior to placebo for more than two days longer than bupivacaine. One of the formulations remained statistically superior to placebo for more than four days. Further, as NaV1.7 does not have an impact on mobility, this approach may offer a better option for post-surgical physical therapy as current nerve block therapies cause temporary paralysis in the affected area.
Similarly for the mechanical allodynia test results, three of the four formulations showed statistically better efficacy for a longer duration of time than bupivacaine. The mechanical allodynia test is shorter in duration, reflecting the subject’s innate swift recovery rate to surgical incisions. Nonetheless, the results mirrored the successful results set forth with the thermal hyperalgesia test.
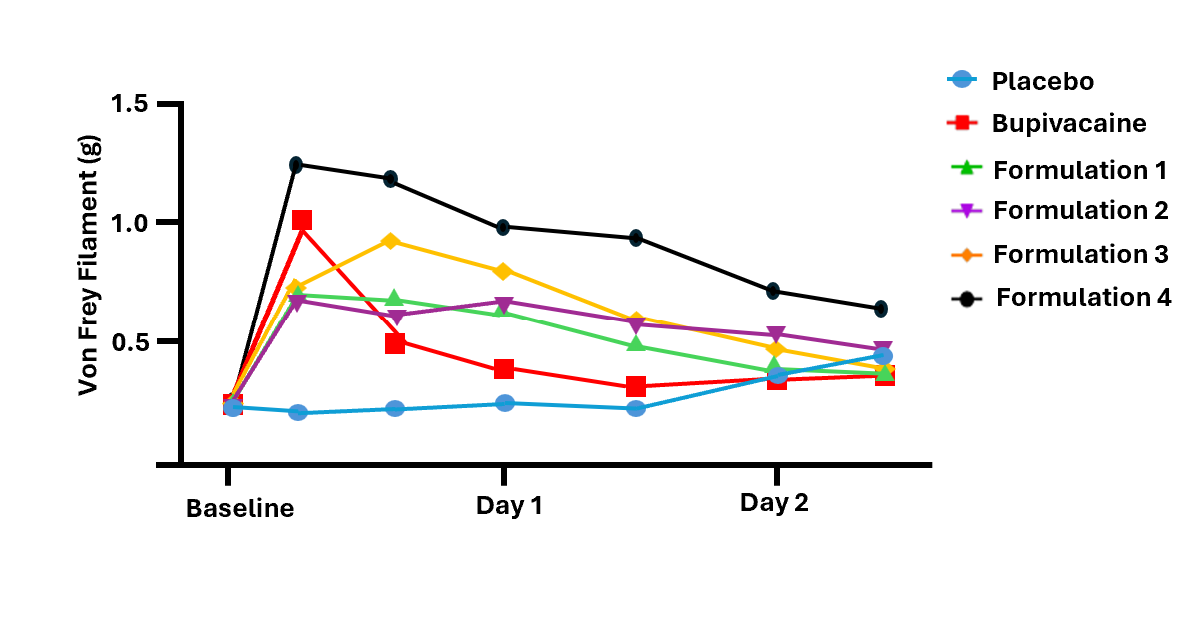
“These results support our belief that NaV1.7 is a potent and effective target for the development of drugs for the treatment of pain,” stated Frank Knuettel II, Chief Executive Officer of Channel. “With these results, investors should feel encouraged that we have a strong development path towards successfully launching drugs with considerable market opportunities,” Mr. Knuettel concluded.
According to BioSpace, the post-operative global pain market was valued at $2.6 billion in 2023, of which, Exparel, a bupivacaine liposome injectable suspension, generated approximately $538 million in revenue. It is this existing market that the Company is tackling, as well as the post-surgical opioid market opportunity, as these results show potential efficacy for a long period of time, which should reduce post-surgical opioid usage.
Results are expected for the studies conducted on the Company’s eye drops for the treatment of various types of eye pain – including severe dry eye, corneal abrasions, surgical intervention and other indications – in late January 2025.
About Channel
Channel Therapeutics Corporation is a clinical-stage biotechnology company focused on developing and commercializing novel, non-opioid, non-addictive therapeutics to alleviate pain. The Company’s initial clinical focus is to selectively target the sodium ion-channel known as NaV1.7 for the treatment of various types of chronic pain, acute and chronic eye pain and post-surgical nerve blocks. For company updates and to learn more about Channel, visit www.channeltherapeutics.com or follow us on social media.
Forward-Looking Statements
This press release contains forward-looking statements regarding the Company’s current expectations. These forward-looking statements include, without limitation, references to the Company’s expectations regarding (i) the Company’s belief that nerve blocks with its NaV1.7 inhibitors may be viable options for the treatment of acute and postoperative pain, (ii) the Company’s belief the Company’s NaV1.7 inhibitor has the potential to improve on existing postoperative therapeutic options while opening the door for success with the Company’s other programs, (iii) the Company’s belief that the NaV1.7 inhibitor may offer a better option for post-surgical physical therapy as compared to bupivacaine, (iv) the Company’s belief that investors should feel encouraged that the Company has a strong development path towards successfully launching drugs with considerable market opportunities, and (iv) the timing of expected results on the Company’s eye drops for the treatment of various types of eye pain. These statements are not guarantees of future performance and are subject to certain risks, uncertainties and assumptions that are difficult to predict. Factors that could cause actual results to differ materially from those set forth in such forward-looking statements include, but are not limited to, risks and uncertainties related to there being no guarantee that the trading price of the Company’s Common Stock will be indicative of the Company’s value or that the Company’s Common Stock will become an attractive investment in the future. These and other risks and uncertainties are described more fully in in our filings with the U.S. Securities and Exchange Commission. The information in this press release is provided only as of the date of this press release, and we undertake no obligation to update any forward-looking statements contained in this press release based on new information, future events, or otherwise, except as required by law.
Channel Media and Investor Inquires:
For Investor Inquiries:
Mike Moyer
Managing Director, LifeSci Advisors, LLC
[email protected]

